Imagine there’s a leak in the roof of your house that you can’t stop. As soon as you make one repair, the leaks start coming from another spot. Eventually, you’ll get exhausted trying to keep up.
It turns out that a similar scenario can play out in your cells as you grow older or after you suffer a heart attack or a stroke.
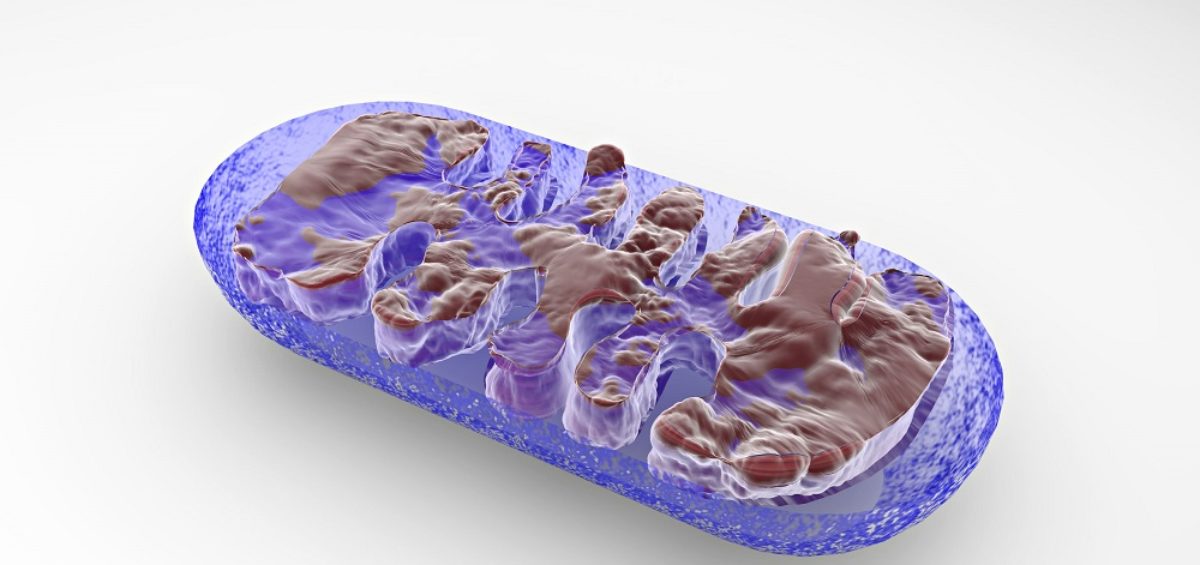
In a recent study published in Cell, a Massachusetts General Hospital research team led by Alexander Soukas, MD, PhD, detailed a paradigm-shifting discovery in which an increase in the permeability of mitochondria in cells elevates levels of autophagy—a typically beneficial process of clearing out old or damaged cell parts and molecules—rendering the process harmful rather than helpful.
The findings could improve our understanding of age-related dysfunction and point to new ways to reduce the tissue damage that occurs when blood supply is cut off to the heart or brain during a heart attack or stroke.
“We have found for the first time that the ‘leakiness’ or permeability of mitochondria determines whether autophagy will extend or shorten lifespan,” says Soukas, an investigator in the Center for Genomic Medicine and Weissman Family MGH Research Scholar 2018-2023.
About Autophagy
High levels of autophagy have long been considered as beneficial to the health of cells because it enables the cell to recycle and reuse parts that are damaged or are no longer useful to it. In animal models in which lifespan is extended through genetic modifications or drugs, high-levels of autophagy are key to that longevity.
However, in some cases, autophagy can be a bad thing. The cell can break down helpful parts as well as dysfunctional ones, which eventually impairs cellular functioning.

How Mitochondrial Permeability Plays A Role
To learn more about the line between harmful and helpful autophagy, Soukas’ team took a closer look at genes that have been linked to the process. They found a mouse model with a mutated SGK gene in which autophagy levels were high but its lifespan was shorter.
“What that said to us is that it’s not autophagy levels per se, but something the autophagy is doing in the cellular context that makes it helpful or harmful,” Soukas explains.
“Whenever you see something like that which is going in the opposite direction of what is almost accepted dogma in the field, that’s science telling you to pay attention and figure out why.”
The findings suggested that the SGK molecule plays a key role in regulating mitochondrial permeability, and if it is lacking or not functioning correctly, then permeability increases. This increase typically occurs when the mitochondria are damaged, which signals the cell to clear them out and replace them with new ones.
However, if the permeability increases to the point where all mitochondria—damaged and healthy alike—are signaling to the cell that they need to be cleared out, then autophagy becomes a runaway process.
“If you are unable to keep the mitochondria from becoming leaky, autophagy gets turned into a destructive force,” Soukas explains. “The idea is basically that the cell is spinning wheels. It’s spending futile resources to try to catch up and is unable to do that.”
“If you can reverse that high autophagy level a little bit, the cells can catch up and you wind up making these animals healthier.”
Reducing Damage After Heart Attacks and Strokes
Autophagy is believed to contribute to ischemia-reperfusion injury—tissue damage that occurs when blood flow returns to the heart or brain after it has been blocked off by a heart attack or stroke.
Soukas and his team found that mice lacking the SGK gene were more susceptible to ischemia reperfusion injury than mice with a functioning copy of the gene. They also found it was possible to reduce the extent of the injury by using a drug that can close mitochondrial pores.
“We think our work has major implications for preventing ischemic injury during heart attack and stroke, as well as during organ transplantation, which requires temporary interruption of the donor’s blood supply,” says Soukas.
“Targeting mitochondrial permeability has great potential for reducing the devastating outcome of these occurrences.”
MGH Research Scholar Award Provides Crucial Support
Soukas says the flexible funding provided by his MGH Research Scholar award ($100,000 per year in unrestricted funding for five years) played a key role in his findings.
“The award allows you to leverage philanthropic dollars to expand the creativity of your research program,” he explains. “We took more time and higher risks to bring this study to fruition, and that wouldn’t have been possible without that support.
About the Mass General Research Institute
Research at Massachusetts General Hospital is interwoven through more than 30 different departments, centers and institutes. Our research includes fundamental, lab-based science; clinical trials to test new drugs, devices and diagnostic tools; and community and population-based research to improve health outcomes across populations and eliminate disparities in care.
Support our Research
COVID-19 Research at Mass General
Researchers and clinicians at Massachusetts General Hospital Research Institute are mobilizing to develop new strategies to diagnose, treat and prevent COVID-19. Learn more.





Leave a Comment